Introduction: Reduced intensity conditioning hematopoietic bone marrow stem cell transplantation (HSCT-RIC) is the only curative option for many hematological malignancies in older/unfit patients. Strategies to minimize complications such as graft versus host disease (GVHD) in HSCT include donor graft engineering but this approach has not been widely tested to the RIC setting due to a potential trade-off between engraftment and reduced GVHD with T cell depletion strategies. Here we present the interim results of our single center Phase I trial evaluating the T cell reduced Orca-T cell therapy product and single agent tacrolimus in the RIC setting.
Methods: The trial is a single-center open-label phase 1 feasibility and safety study for dose escalation of conventional T cell (Tcon) infusion. Primary objectives included measuring the incidence of grade III-IV acute GVHD, time of engraftment and donor T cell chimerism at day +30. Secondary objectives are measuring relapse free survival, severity of GVHD and incidence of serious infections. While the study has three independent trial arms depending on donor type (HLA matched related and unrelated, 9/10 HLA matched or haploidentical matched), accrual has focused on the HLA-matched cohort which is reported here. The first 11 patients on this arm were conditioned with fludarabine 40mg/m 2, melphalan 50 mg/m 2 and 4 Gy of total body irradiation (TBI), an additional 4 patients have been treated with thiotepa 10 mg/kg replacing the melphalan. All patients received single agent tacrolimus with a target goal of 6-8 ng/ml.
Results: A total of 15 HLA-matched patients have been recruited thus far in this interim dose escalation analysis. The median patient age is 68.0 years (range 62 to 72), with 73% male (11/15) patient distribution. Pre transplant, 53% had acute myeloid leukemia (8/15), 20% had myelodysplastic syndrome (3/15), 13% had acute lymphoblastic leukemia (2/15) and 13% had a myeloproliferative neoplasm (2/15). Prior to transplant, all acute leukemia patients were in complete remission (CR) or CR with incomplete count recovery.
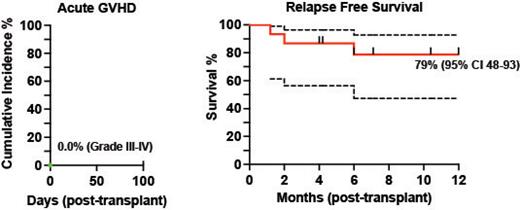
Median follow up was 10.4 months for the entire cohort (range 2 to 20 months). 14/15 patients had engraftment by day 20 and 1/15 at day 39 post-transplant. Based on these findings, no dose escalation of Tcon infusion was needed. None of the patients had infusion reactions on day of transplant nor engraftment syndrome. Median percent donor chimerism for CD15 was high, with 100% at day 30 (15/15, range 94% to 100%) and 99% (13/15, patients with available data, range 98% to 100%) at day 90. Median CD3 chimerism at days 30 and 90 of transplant were also high at 99% (15/15, range 68% to 100%) and 99% (for 13/15 patients with available data, range 78% to 100%) respectively. No patients developed grade III-IV acute GVHD ( Fig.), defined <100 days post-transplant. One patient developed grade II aGVHD. In 12 months, the incidence for moderate to severe chronic GVHD was 7%. The 12-month relapse free survival (RFS) is thus far 79% ( Fig.). The cumulative incidence for grade 2 and 3 infections in the first 90 days was 26.7%, where 2 patients died. One patient died at day 180 from liver biopsy complications for late onset hepatic sinusoidal obstruction syndrome. No surviving patient has had disease relapse.
Conclusions: These early results demonstrate that Orca-T is a safe and feasible transplant strategy in the RIC setting for patients with advanced hematological malignancies. Patients showed robust and early donor myeloid and eventual full donor T cell engraftment. The low incidence of acute and chronic GVHD with low rates of disease relapse, suggest retention of graft versus leukemia effect. Few studies of donor graft-engineered products have been successfully evaluated in the RIC setting and merits larger multicenter studies evaluatingGVHD-free and RFS post-transplant to validate these findings.
Disclosures
Meyer:Orca Bio: Research Funding. Negrin:Garuda Therapeutics: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Biorasi: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Appia Bio: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Co-Immune: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; UpToDate: Patents & Royalties; Regimmune, Inc.: Consultancy; Cellenkos: Consultancy; Orca Bio: Research Funding. Frank:Roche/Genentech: Current holder of stock options in a privately-held company; Allogene: Consultancy; Kite, a Gilead Company: Research Funding; Adaptive Biotechnology: Consultancy; EcoR1: Consultancy; BRVLH: Consultancy; Cargo Therapeutics: Consultancy, Other: Travel Support; Gilead Sciences: Consultancy, Other: Travel Support. Lowsky:Orca Bio: Research Funding. Miklos:MorphoSys: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Mustang Bio: Consultancy, Honoraria; Kite, a Gilead Company: Consultancy, Research Funding; 2Seventy Bio: Research Funding; Bioline Rx: Membership on an entity's Board of Directors or advisory committees; NA: Patents & Royalties: cGVHD patent holder for Ibrutinib as cGVHD therapy but no compensation; Legend Biotech: Consultancy, Honoraria; Juno Therapeutics: Consultancy, Honoraria, Patents & Royalties: rights to royalties from Fred Hutch for patents licensed to Juno, Research Funding; Bristol-Myers Squibb: Consultancy; Genentech: Consultancy, Honoraria; Gilead Sciences: Consultancy, Honoraria; Adaptive Biotechnologies: Consultancy; Janssen: Consultancy, Honoraria, Other: Travel support; Miltenyi: Consultancy, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria; A2 Biotherapeutics: Consultancy, Current holder of stock options in a privately-held company, Honoraria; Fate Therapeutics: Research Funding; Allogene: Research Funding; Adicet: Research Funding; Navan Technologies: Consultancy, Current holder of stock options in a privately-held company, Honoraria; Umoja: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria. Muffly:kite: Consultancy, Honoraria, Research Funding; pfizer: Consultancy; amgen: Consultancy; jasper: Research Funding; astellas: Consultancy, Research Funding; autolus: Consultancy; orca bio: Research Funding; bms: Research Funding; adaptive: Membership on an entity's Board of Directors or advisory committees, Research Funding. Dahiya:Bristol Myers Squibb: Consultancy; Incyte: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Adaptive Biotechnologies: Consultancy. Rezvani:Pharmacyclics.: Research Funding. Sidana:Magenta Therapeutics, BMS, Janssen, Sanofi, Oncopeptides, Takeda, Pfizer: Consultancy; Magenta Therapeutics, BMS, Allogene, Janssen, Novartis: Research Funding. Shizuru:Jasper Therapeutics: Consultancy, Current equity holder in publicly-traded company. Smith:A28: Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy. Pavlova:Orca Bio: Current Employment. McClellan:Orca Bio: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal